2025 Year in Review: A Message from Our Network Chairs
Dear IMPAACT Network Colleagues,
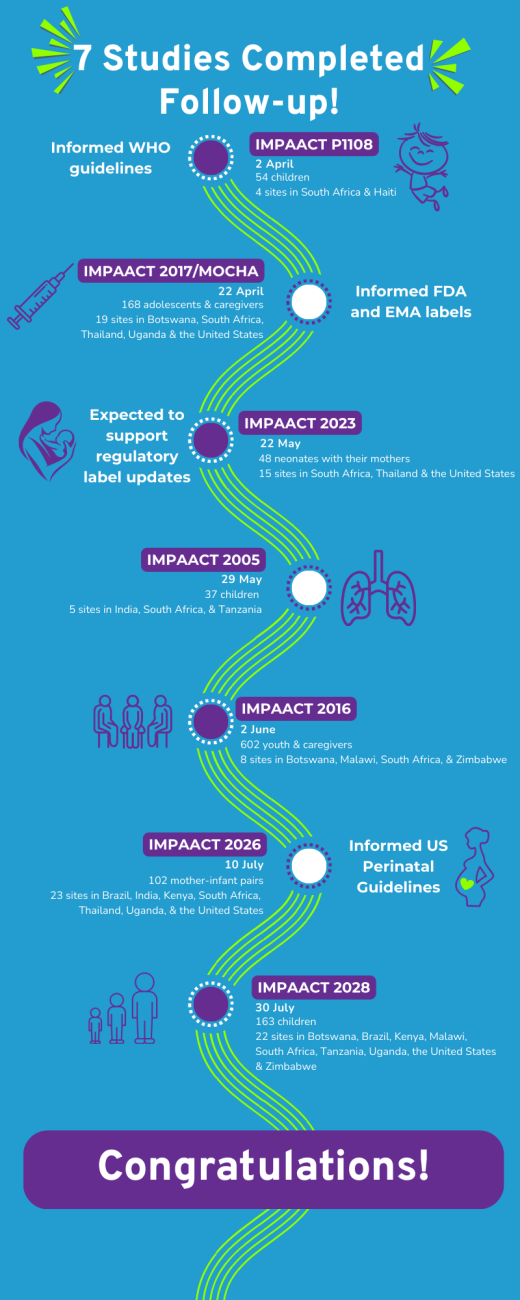
As we approach the end of the year, we are reflecting on our shared accomplishments and renewing our commitment to the work that still lies ahead. Throughout 2025, the IMPAACT Network has navigated challenges and ongoing changes, adapting to the everchanging public health landscape while continuing to deliver on our mission: improving health outcomes in infancy, childhood, adolescence, pregnancy, and postpartum among those who are impacted by or living with HIV. The resiliency of our Network has led to vital achievements in our efforts to end the HIV epidemic. In the past year, four protocols were finalized (IMPAACT 2024, 2041, 2044, and 2046/UPLIFT), two studies opened to accrual (IMPAACT 2037 and 2041), and seven studies closed to follow-up (P1108, IMPAACT 2005, 2016, 2017/MOCHA, 2023, 2026, and 2028), with highlights below. More information about these studies can be found on the IMPAACT website: https://www.impaactnetwork.org/. These milestones underscore the continued importance of our studies and our ability to persevere in adverse conditions.
In January, the Scientific Leadership Group (SLG) named Dr. Jennifer Jao as Network Co-Chair Elect to serve alongside Dr. Sharon Nachman. Dr. Jao has been instrumental in this role as we navigated the year’s ups and downs and guided the Network forward.
The Network announced the 2025 Early Career Investigators (ECI) in February. This class of ECIs has spent the year working on their respective projects with Network mentors and will be presenting their findings at next year’s Annual Meeting. Their projects include assessing the effects of immune suppression of pregnancy on the HIV latent reservoir, the role of social factors on infant outcomes, and systemic inflammation in pregnant and postpartum mothers with HIV and their children.
In March, data from IMPAACT 2017/MOCHA informed the European Commission’s authorization of ViiV Healthcare’s long-acting injectable Vocabria (cabotegravir long-acting injections) and Johnson and Johnson’s Rekambys (rilpivirine long-acting injections). The authorization expands the adult approvals of long-acting cabotegravir and rilpivirine for HIV treatment to adolescents aged 12 years and older and weighing at least 35 kg who are virologically suppressed.
Network researchers also participated in the 2025 Conference on Retroviruses and Opportunistic Diseases (CROI) in San Francisco, California, USA by presenting data during five poster presentations and one themed discussion. Data from IMPAACT 2010/VESTED, P1026s, PROMISE P1084s, IMPAACT 2036/CRAYON, and IMPAACT 2023 were presented. View the presentations here.
In May, IMPAACT 2023, a Phase I study of safety, tolerability, and pharmacokinetics of dolutegravir in neonates exposed to HIV, completed participant follow-up. Forty-eight mother-infant pairs were enrolled across sites in South Africa, Thailand, and the United States. The completion of this study represents a key step toward supporting licensure of dolutegravir for use in infants during the first four to six weeks of life.
IMPAACT was well-represented at the International AIDS Society (IAS) 2025 meeting and the 13th Conference on HIV Science and the HIV Pediatrics Workshop held in Kigali, Rwanda in July. Colleagues presented two posters at IAS 2025 from IMPAACT 2009 and 2016, while the Peds Workshop included two oral presentations from IMPAACT 2009, and two posters from 2009 and 2016. View the presentations here.
In September, the IMPAACT Network Annual Meeting was held virtually with over 600 attendees participating in the Community Advisory Board and Network plenary sessions. Later that month, results from IMPAACT P1115 were published in The Lancet HIV highlighting that four children remained free of detectable HIV for more than one year after their antiretroviral therapy was paused to see if they could achieve HIV remission. Dr. Deborah Persaud, virologist for P1115, and Chair of the IMPAACT Cure and Immunotherapy Scientific Committee, discussed the study’s findings and the possibility of HIV remission in neonates on The Lancet HIV in conversation with podcast.
In November, the IMPAACT 2034 team presented data at the 2025 Union World Lung Health Conference, showing that a single dose of pretomanid was safe, well-tolerated, and achieved pharmacokinetic (PK) exposures comparable to adults in female children with rifampicin-resistant tuberculosis. You can read more about the study’s findings here.
We would like to recognize and thank those who bring this important work to fruition. The daily work of our clinical research site staff, community members, investigators, study participants and their caregivers/parents, central Network staff, committee and protocol team members, pharmaceutical collaborators, sponsors, and other Network partners is the foundation of our success. We are grateful for your resolute commitment to our mission.
Now—more than ever—IMPAACT remains steadfast in our promise to continue working on behalf of the community we serve to help those affected by HIV through infancy, childhood, adolescence, pregnancy, and postpartum.
We wish you happy holidays and much joy in the new year!
Sincerely,